Abstract
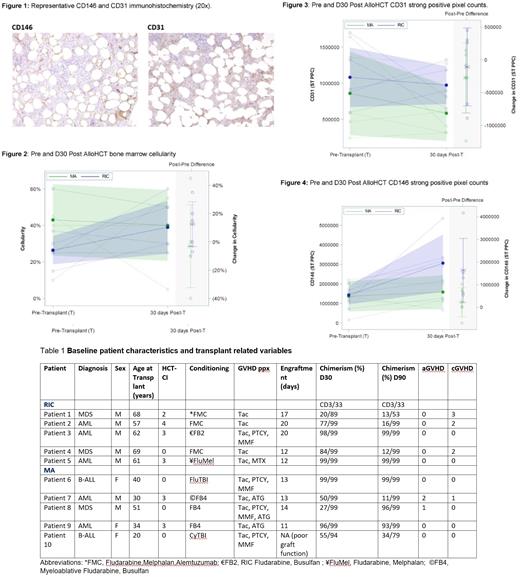
Timely and effective reconstitution of the hematopoietic niche is imperative post-allogeneic stem cell transplantation (alloHCT) for prevention of life-threatening opportunistic infections and relapses. Efficient engraftment is dependent on the ability of donor hematopoietic stem cells (HSCs) to expand and provide long-term multi-lineage progenitors in the recipient. Mesenchymal stem cells (MSCs) are essential in providing a conducive environment for HSC reconstitution and maintenance. Conditioning chemotherapy with or without total body irradiation is required to confer a competitive advantage to donor HSCs. However, myeloablative conditioning (MA) regimens also destroy the specialized stromal niches which support HSC self-renewal and maintenance (Acar et al., 2015), providing a plausible explanation for the long term cytopenias observed post allogeneic transplantation (Domenech et al., 1995). Previous studies in humans showed CD146-expressing stromal cells support the establishment of the hematopoietic cell microenvironment (Sacchetti et al., 2007). Expression of markers of stromal/progenitor cells (CD146, CD31 and CD34) was evaluated pre and post alloHCT at day 30 in bone marrow biopsies obtained from patients treated with either MA or reduced intensity conditioning (RIC) approaches. CD146 and CD31 expression was evaluated by immunohistochemistry performed on formalin-fixed, paraffin-embedded bone marrow core biopsies. Whole slide imaging (Aperio) was used to digitize the histology and positive cells were scored using image analysis software (strong positive pixel count; ST PPC). In bone marrow, CD146 is expressed on vascular endothelial cells, pericytes on sinusoids, adipose and bone marrow stromal cells. CD31 positive cells include vascular endothelial cells, sinusoidal cells, megakaryocytes, and myeloid cells (Fig. 1). The percent of CD34 positive stem cells was determined by flow cytometry in concurrent bone marrow aspirate cells.
The indications for an alloHCT were AML (5/10), ALL (2/10) or MDS (3/10). Median age was 62 (59-69) years in the RIC group and 34 (25-46) years in the MA group. Average HCT CI score was higher in the RIC group (2.5 versus 1.2) (Table 1). 3/10 patients received total body irradiation (TBI) based conditioning. All patients received peripheral blood stem cell alloHCT and 7/10 were MUDs. Chronic GVHD was present (grade 2 or 3) in 3/10 patients in the RIC groups and 1 patient (grade 1) in the MA cohort. Chimerism studies showed optimum engraftment of donor cells in 2 patients in the RIC group and 1 patient in the MA (Table 1).
On average, pretransplant bone marrow cellularity was lower in the RIC group. The average overall cellularity on D30 was similar in the two groups (Fig.2). The average percent of CD34 positive cells was similar pretransplant and lower at D30 in the RIC cohort compared to the MA group (0.96% CD34 pre alloHCT versus 0.30% D30 post alloHCT RIC; pre alloHCT 0.96% CD34 versus D30 post alloHCT 0.92% MA). The mean number of CD31 positive cells was higher in the RIC group compared to the MA cohort both pre-transplant and at D30 (Fig 3). On average, an increase in CD146 positive cells on D30 was noted in the alloHCT RIC group compared to the MA cohort (Fig. 4).
In conclusion, the RIC cohort showed a pre-post-transplant increase in average overall bone marrow cellularity, more numerous CD31 and CD146 positive cells on average and an increase in CD146 positive cells at D30 post alloHCT compared to the MA group. Despite the modest number of patients, this is the first study to date comparing pre and post alloHCT niche components which may provide important insights in the reconstitution process and impact of conditioning.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal